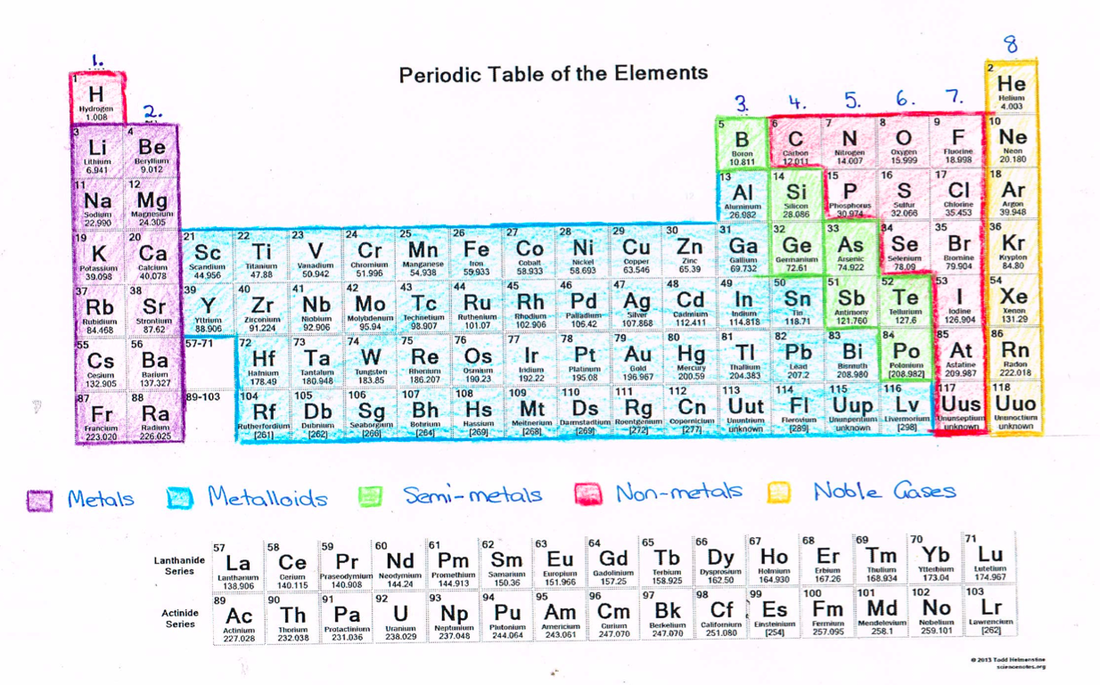
Special Metals Periodic Table . Also, the two rows of elements. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. This describes groups 3 through 12 on. Elements that are not metals include the. As shown in figure \(\pageindex{2}\),. the largest group of elements on the periodic table is that of the transition metals, found in the middle of the table. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. It is obtained chiefly from the mineral cassiterite, which. moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13.
from sciencewithpizzi.weebly.com
As shown in figure \(\pageindex{2}\),. Elements that are not metals include the. This describes groups 3 through 12 on. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Also, the two rows of elements. the largest group of elements on the periodic table is that of the transition metals, found in the middle of the table. It is obtained chiefly from the mineral cassiterite, which. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides.
1.2) Atoms and the Periodic Table Science with Mrs Pizzimenti
Special Metals Periodic Table transition metals are defined as those elements that have (or readily form) partially filled d orbitals. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. the largest group of elements on the periodic table is that of the transition metals, found in the middle of the table. moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. This describes groups 3 through 12 on. Elements that are not metals include the. As shown in figure \(\pageindex{2}\),. It is obtained chiefly from the mineral cassiterite, which. Also, the two rows of elements.
From bucarotechelp.com
Brief Description of the Chemical and Physical Properties of Elements Special Metals Periodic Table moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. As shown in figure \(\pageindex{2}\),. the metals consist of the alkali metals, alkaline earths,. Special Metals Periodic Table.
From www.britannica.com
Chemical compound Definition, Examples, & Types Britannica Special Metals Periodic Table moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. This describes groups 3 through 12 on. Also, the two rows of elements. Elements that. Special Metals Periodic Table.
From sciencenotes.org
Free Printable Periodic Tables (PDF and PNG) Science Notes and Projects Special Metals Periodic Table transition metals are defined as those elements that have (or readily form) partially filled d orbitals. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. As shown in figure \(\pageindex{2}\),. moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from. Special Metals Periodic Table.
From glossary.periodni.com
Periodic table Chemistry Dictionary & Glossary Special Metals Periodic Table Also, the two rows of elements. This describes groups 3 through 12 on. It is obtained chiefly from the mineral cassiterite, which. the largest group of elements on the periodic table is that of the transition metals, found in the middle of the table. transition metals are defined as those elements that have (or readily form) partially filled. Special Metals Periodic Table.
From mungfali.com
Full Periodic Table Of Elements Special Metals Periodic Table As shown in figure \(\pageindex{2}\),. It is obtained chiefly from the mineral cassiterite, which. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Elements that are not metals include the. moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group. Special Metals Periodic Table.
From tagdiki.weebly.com
Metals on the periodic table tagdiki Special Metals Periodic Table Also, the two rows of elements. As shown in figure \(\pageindex{2}\),. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. This describes groups 3 through 12 on. It is obtained chiefly from the mineral cassiterite, which. Elements that are not metals include the. the largest group of elements on the periodic table is. Special Metals Periodic Table.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table Special Metals Periodic Table Also, the two rows of elements. This describes groups 3 through 12 on. It is obtained chiefly from the mineral cassiterite, which. the largest group of elements on the periodic table is that of the transition metals, found in the middle of the table. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides.. Special Metals Periodic Table.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Special Metals Periodic Table moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. It is obtained chiefly from the mineral cassiterite, which. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Also, the two rows of elements.. Special Metals Periodic Table.
From animalia-life.club
Periodic Table Metals List Special Metals Periodic Table This describes groups 3 through 12 on. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Also, the two rows of elements. moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. As shown. Special Metals Periodic Table.
From sciencenotes.org
Periodic Table With Everything Special Metals Periodic Table This describes groups 3 through 12 on. moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. It is obtained chiefly from the mineral cassiterite, which. Elements that are not metals include the. As shown in figure \(\pageindex{2}\),. the largest group. Special Metals Periodic Table.
From www.periodictableprintable.com
How To Identify Metals And Non Metals On The Periodic Table Periodic Special Metals Periodic Table moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. the largest group of elements on the periodic table is that of the transition metals, found in the middle of the table. It is obtained chiefly from the mineral cassiterite, which.. Special Metals Periodic Table.
From www.livescience.com
How the Periodic Table groups the elements Live Science Special Metals Periodic Table As shown in figure \(\pageindex{2}\),. the largest group of elements on the periodic table is that of the transition metals, found in the middle of the table. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. It is obtained chiefly from the mineral cassiterite, which. transition metals are defined as those elements. Special Metals Periodic Table.
From schoolbag.info
Figure 2.3. Periodic Table, Coded by Element Type Special Metals Periodic Table moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. It is obtained chiefly from the mineral cassiterite, which. This describes groups 3 through 12 on. As. Special Metals Periodic Table.
From www.musiccrowns.org
Periodic Table of Metal Special Metals Periodic Table moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Elements that are not metals include the. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. the largest group of elements on the periodic table. Special Metals Periodic Table.
From study.com
Periodic Table Metals Definition, Reactivity & Examples Video Special Metals Periodic Table the largest group of elements on the periodic table is that of the transition metals, found in the middle of the table. It is obtained chiefly from the mineral cassiterite, which. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. the metals consist of the alkali metals, alkaline earths, transition. Special Metals Periodic Table.
From www.periodictableprintable.com
Description Of Metals On The Periodic Table Periodic Table Printable Special Metals Periodic Table the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. It is obtained chiefly from the mineral cassiterite, which. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. This describes groups 3 through 12 on. Also, the two rows of elements. the largest group of elements. Special Metals Periodic Table.
From sciencenotes.org
Representative Elements on the Periodic Table Special Metals Periodic Table Elements that are not metals include the. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. This describes groups 3 through 12 on. the largest group of elements on the periodic table is that of the. Special Metals Periodic Table.
From www.alamy.com
Colorful modern background of the periodic table of the chemical Special Metals Periodic Table Also, the two rows of elements. the metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. transition metals are defined as those elements that have (or readily form) partially filled d orbitals. This describes groups 3 through 12 on. As shown in figure \(\pageindex{2}\),. the largest group of elements on the periodic table. Special Metals Periodic Table.